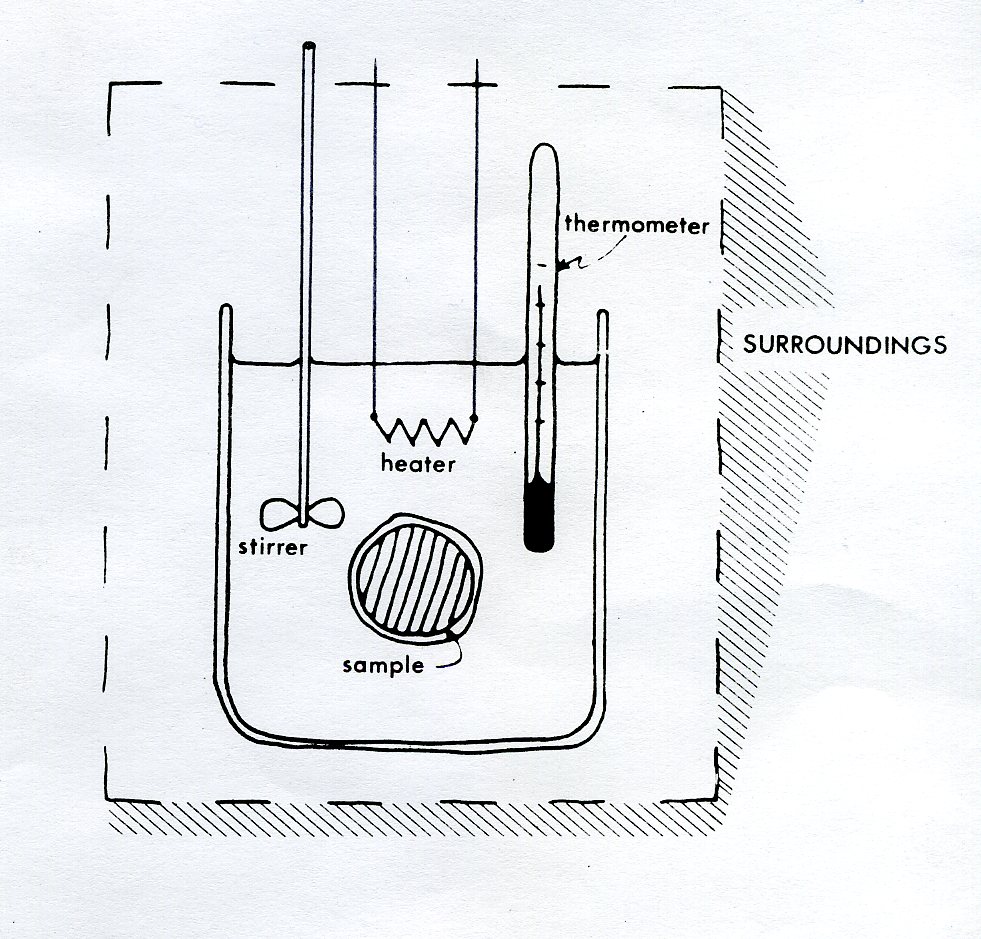
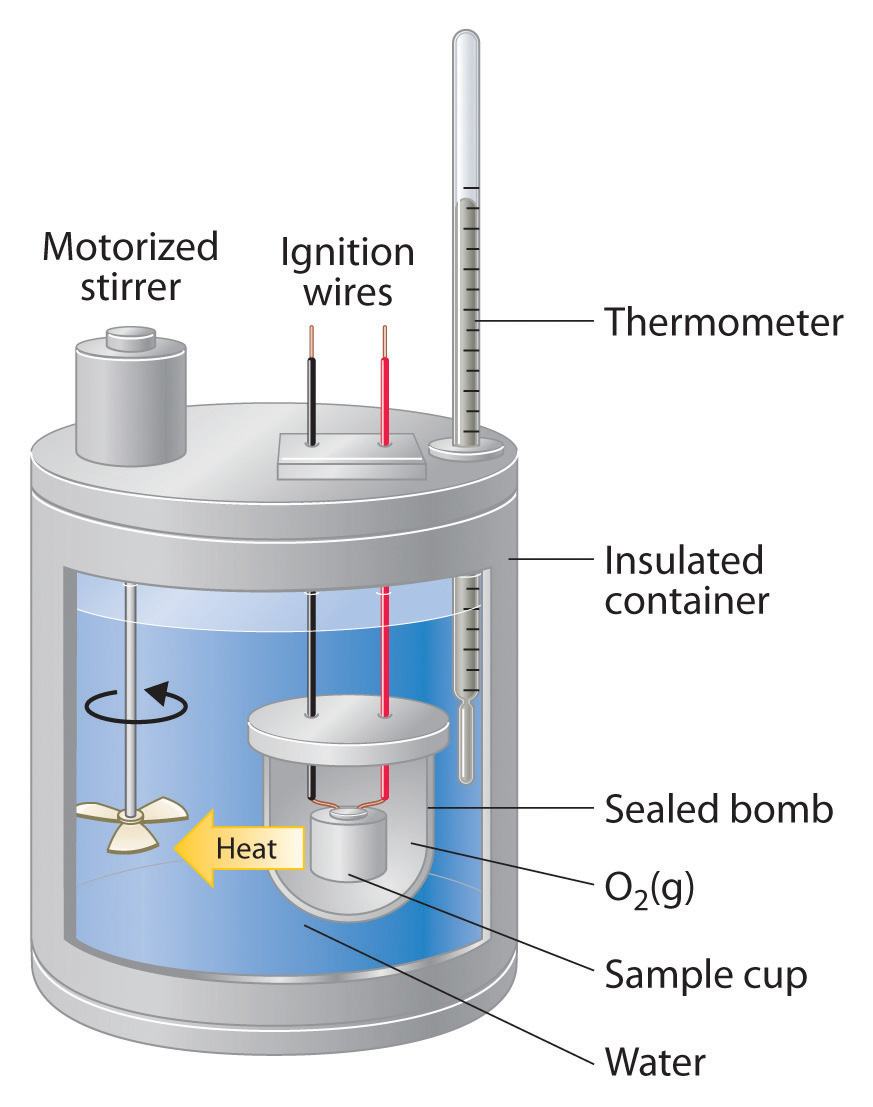
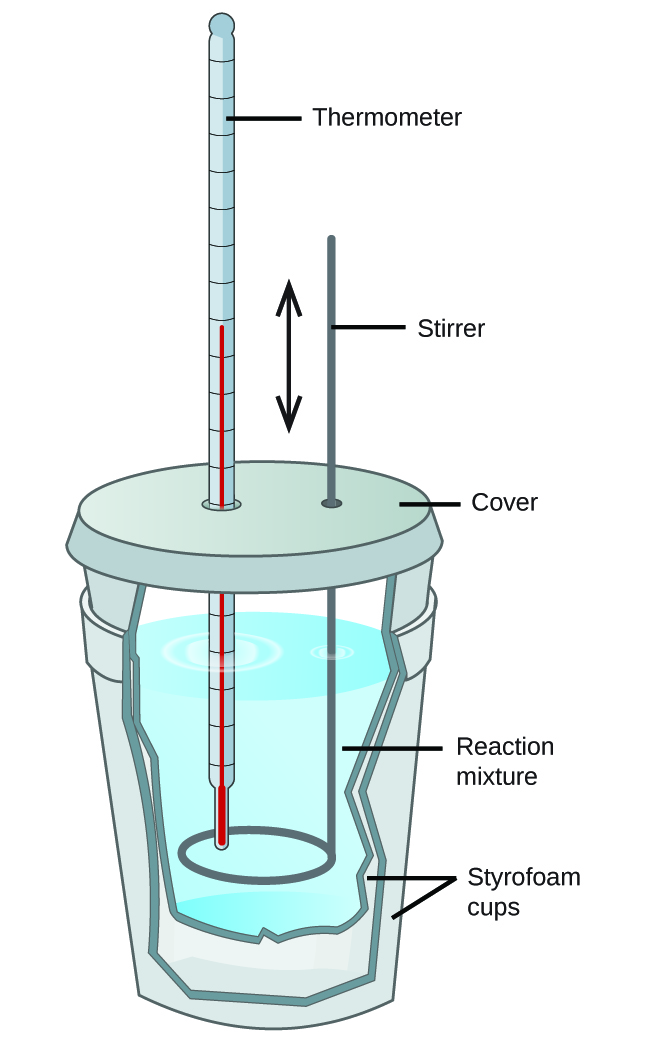
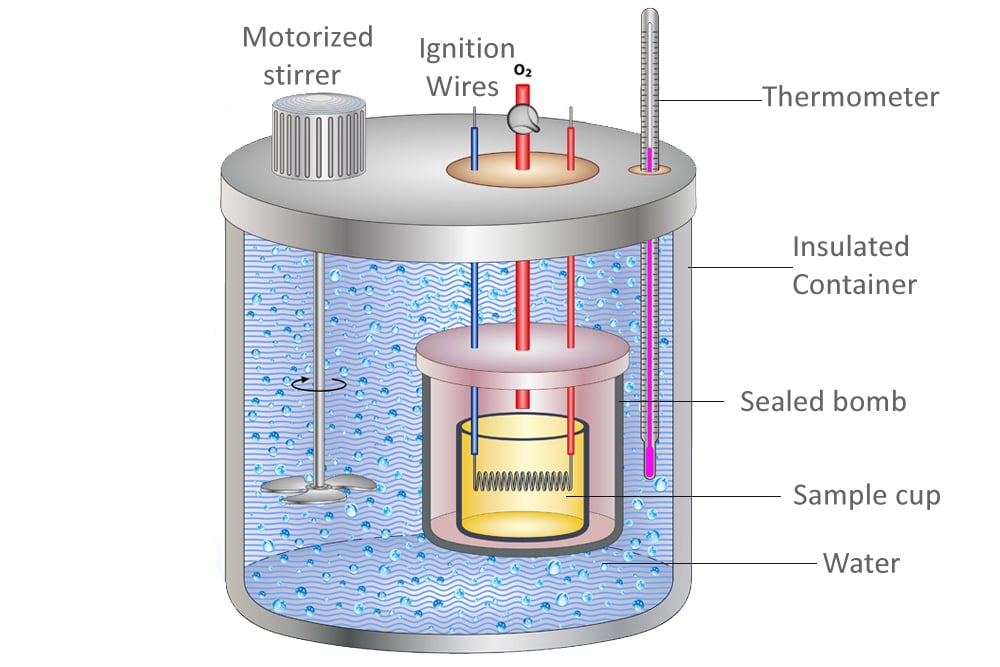
43 how does a calorimeter work
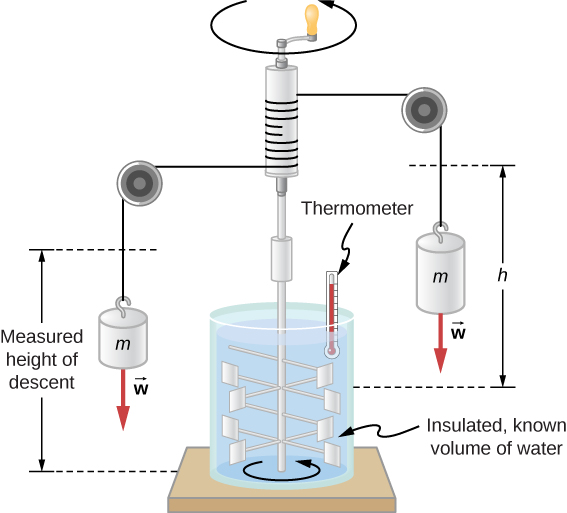
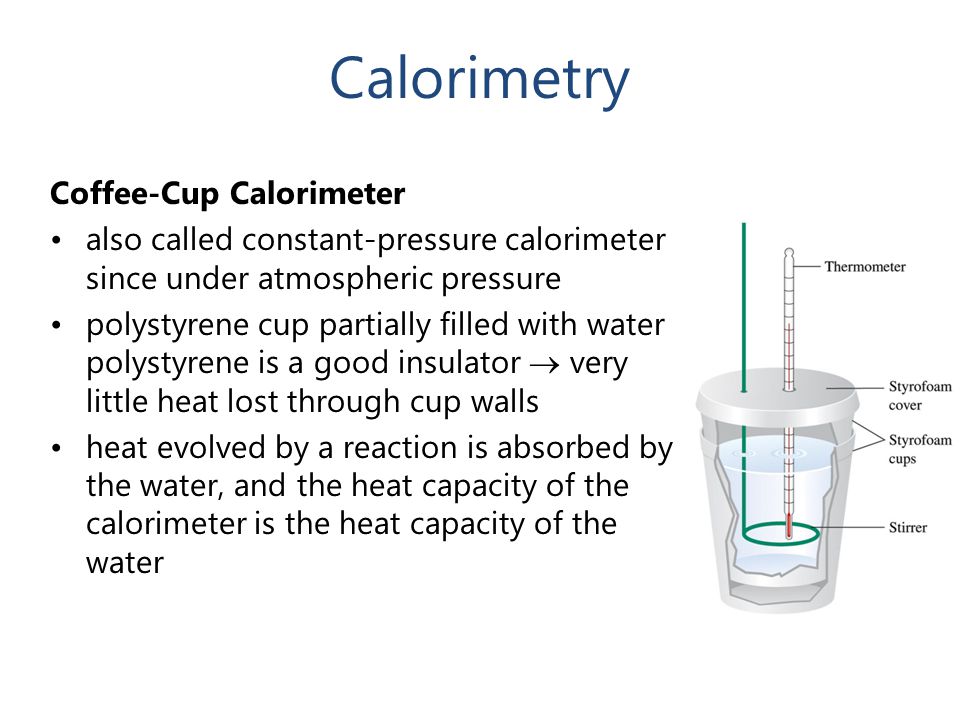
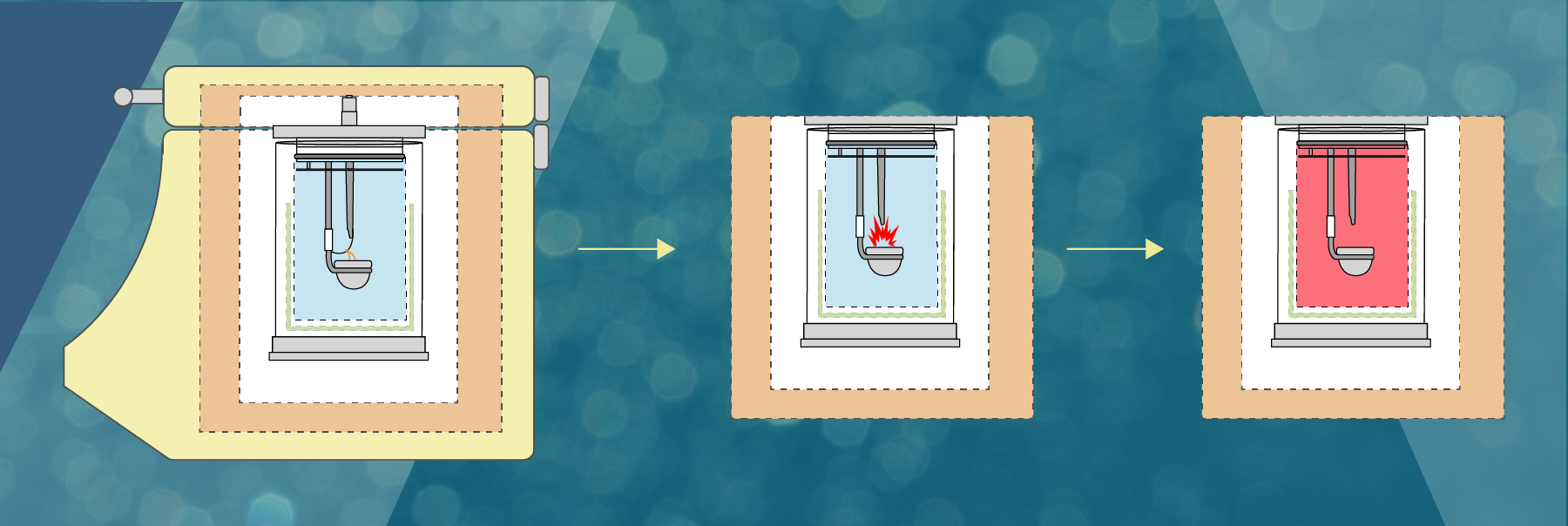
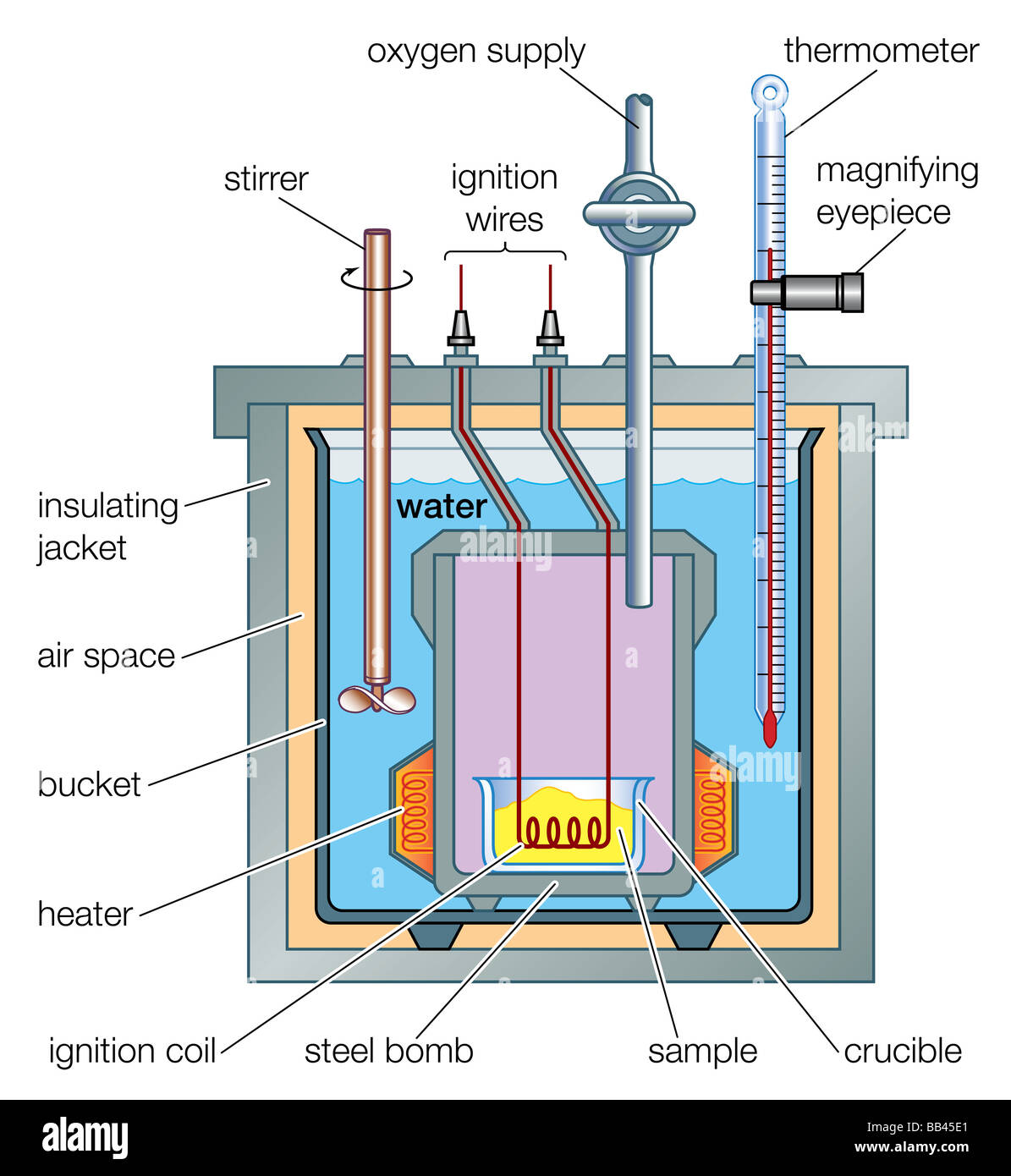
sciencebriefss.com › chemistry › so-how-exactly-doesSo How Exactly Does a Calorimeter Work? - Chemistry ... Jan 5, 2022 · The calorimeter traps all the heat from a chemical reaction, we measure the effect of that heat on the temperature of water in the calorimeter, and we can then calculate the heat energy released by the reaction. The calorimeter is an insulated container, in which we place a measured mass of water. › en › Products-Lab-EqCalorimeters - Frequently Asked Questions - IKA How does a calorimeter work? Calorimetry is the measuring of heat quantities that are linked to biological, chemical or physical processes both exothermic as well as endothermic.IKA offers combustion calorimeters which are distinguishable by their operating modes: adiabatic, isoperibol, or "double-dry".
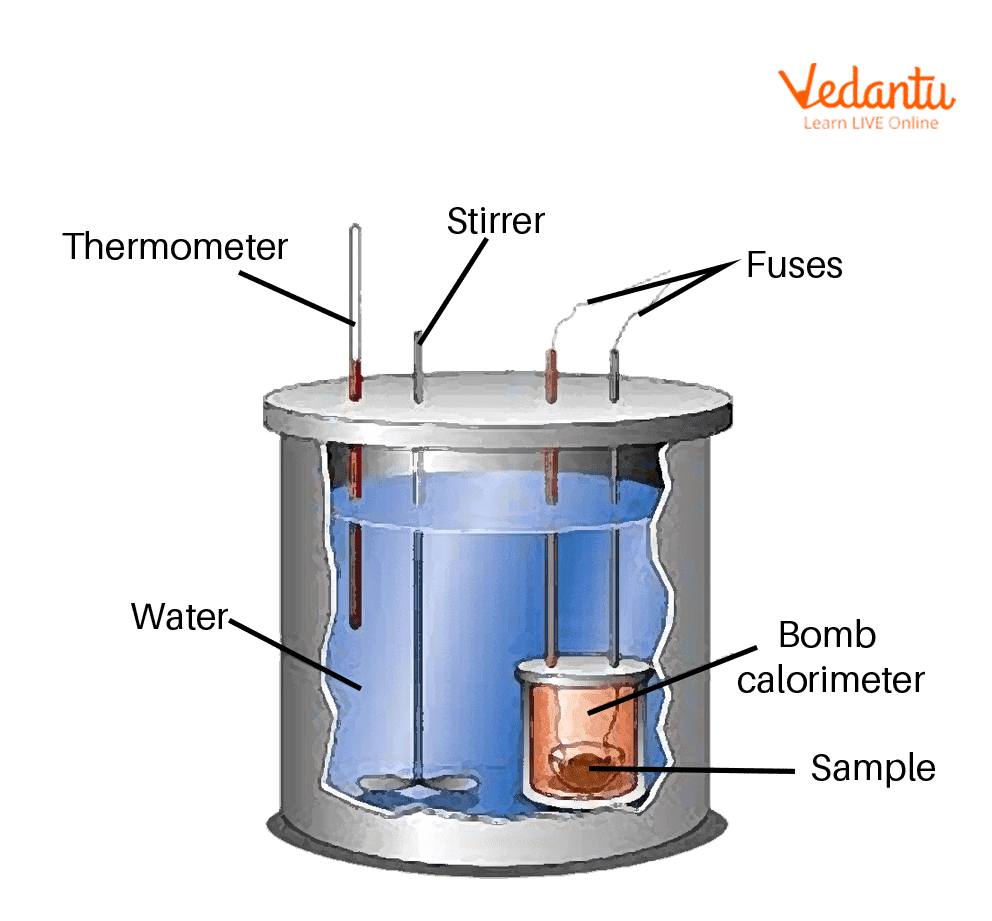
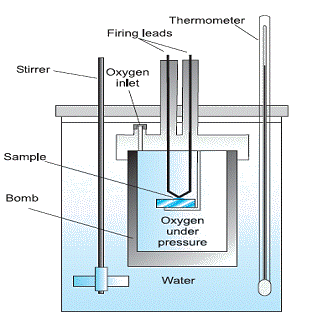
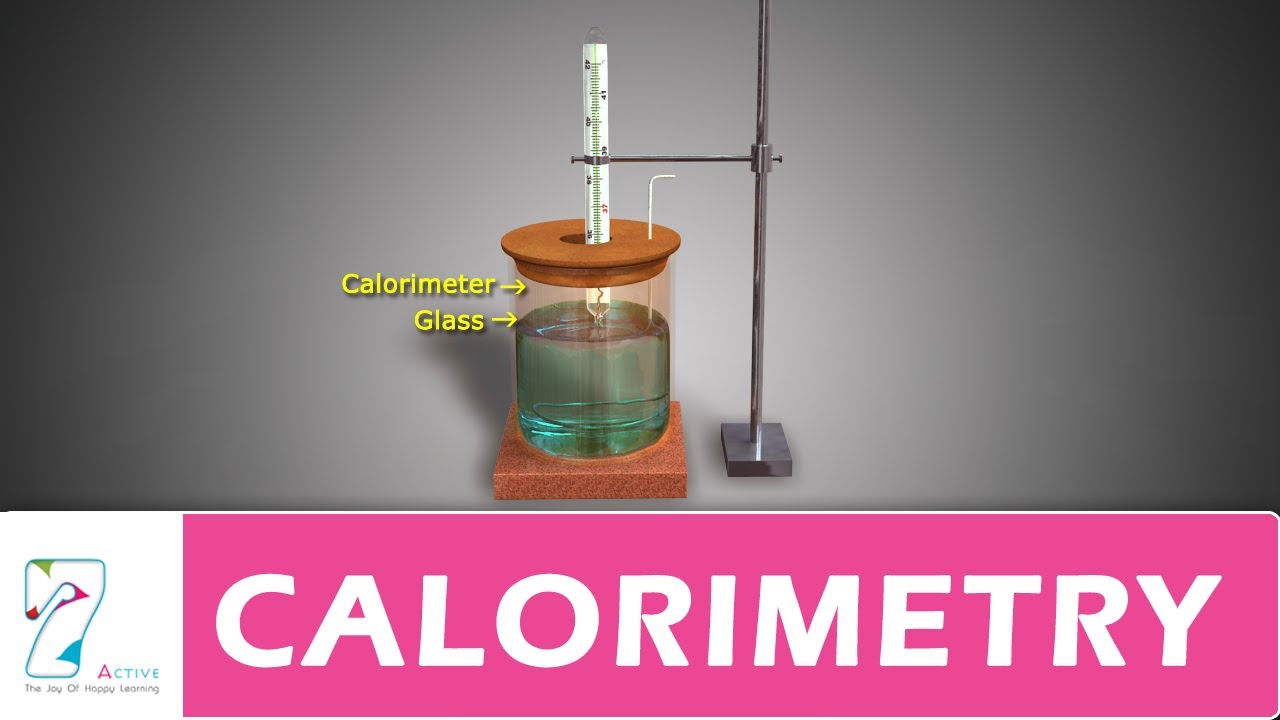
byjus.com › physics › calorimeterCalorimeter - Definition, Uses, Types, Application, Diagram Calorimeter A calorimeter is what is used to measure the thermal changes of a body. Calorimetry is applied extensively in the fields of thermochemistry in calculating the enthalpy, stability, heat...
How does a calorimeter work
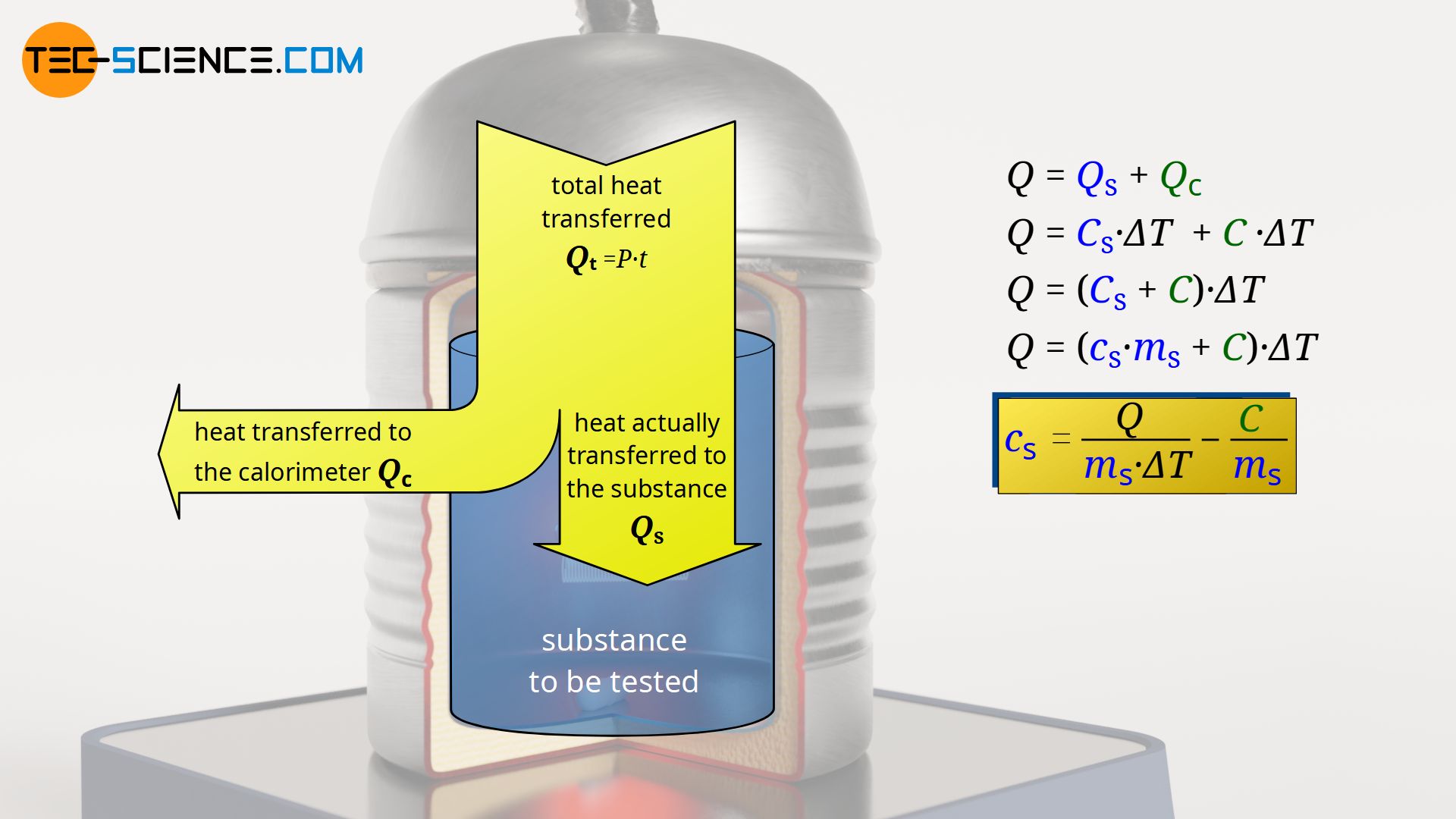
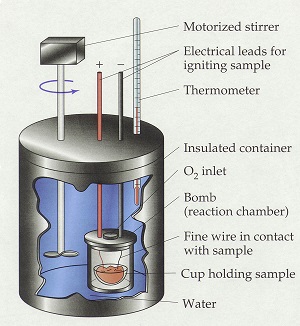
› blog › what-is-a-calorimeterWhat is a Calorimeter & How Is It Used in a Lab? | Excedr Feb 15, 2022 · A calorimeter is a device used for calorimetry, or measuring heat capacity or the heat of physical changes or chemical reactions. In pharmaceuticals, they are used in drug design. In the chemical industry, they are used for quality control, and in biological studies, they are used for metabolic rate examination. chem.libretexts.org › Thermodynamics › CalorimetryCalorimetry - Chemistry LibreTexts Jan 30, 2023 · Calorimetry also plays a large part of everyday life, controlling the metabolic rates in humans and consequently maintaining such functions like body temperature. Constant Pressure Calorimetry Because calorimetry is used to measure the heat of a reaction, it is a crucial part of thermodynamics. › en › methodsDifferential Scanning Calorimetry - How does it work? A typical calorimeter is an isolated chamber where a sample is placed in a surrounding medium. Then the sample is heated with a definite amount of heat. The difference in temperature between sample and surrounding medium gives the heat capacity of the sample and information about heat release and consumption of the sample.
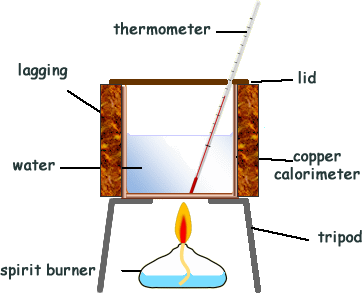
How does a calorimeter work. chem.libretexts.org › Courses › University_of5.6: Calorimetry - Chemistry LibreTexts Feb 14, 2022 · A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. › en › methodsDifferential Scanning Calorimetry - How does it work? A typical calorimeter is an isolated chamber where a sample is placed in a surrounding medium. Then the sample is heated with a definite amount of heat. The difference in temperature between sample and surrounding medium gives the heat capacity of the sample and information about heat release and consumption of the sample. chem.libretexts.org › Thermodynamics › CalorimetryCalorimetry - Chemistry LibreTexts Jan 30, 2023 · Calorimetry also plays a large part of everyday life, controlling the metabolic rates in humans and consequently maintaining such functions like body temperature. Constant Pressure Calorimetry Because calorimetry is used to measure the heat of a reaction, it is a crucial part of thermodynamics. › blog › what-is-a-calorimeterWhat is a Calorimeter & How Is It Used in a Lab? | Excedr Feb 15, 2022 · A calorimeter is a device used for calorimetry, or measuring heat capacity or the heat of physical changes or chemical reactions. In pharmaceuticals, they are used in drug design. In the chemical industry, they are used for quality control, and in biological studies, they are used for metabolic rate examination.






















0 Response to "43 how does a calorimeter work"
Post a Comment