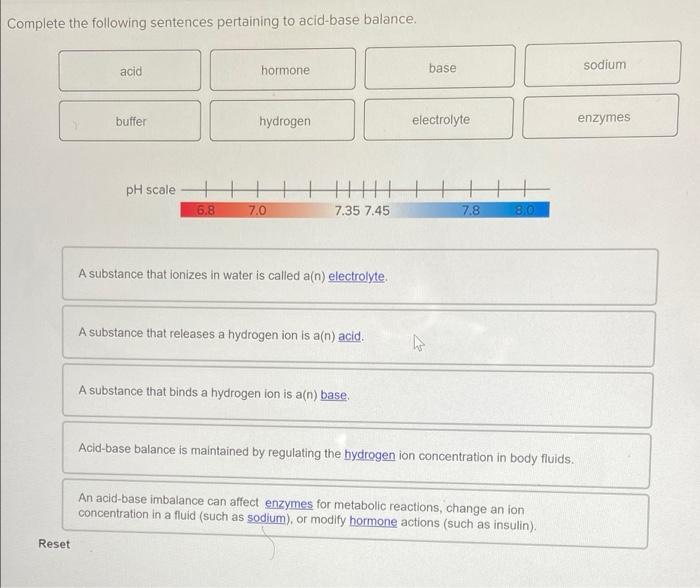
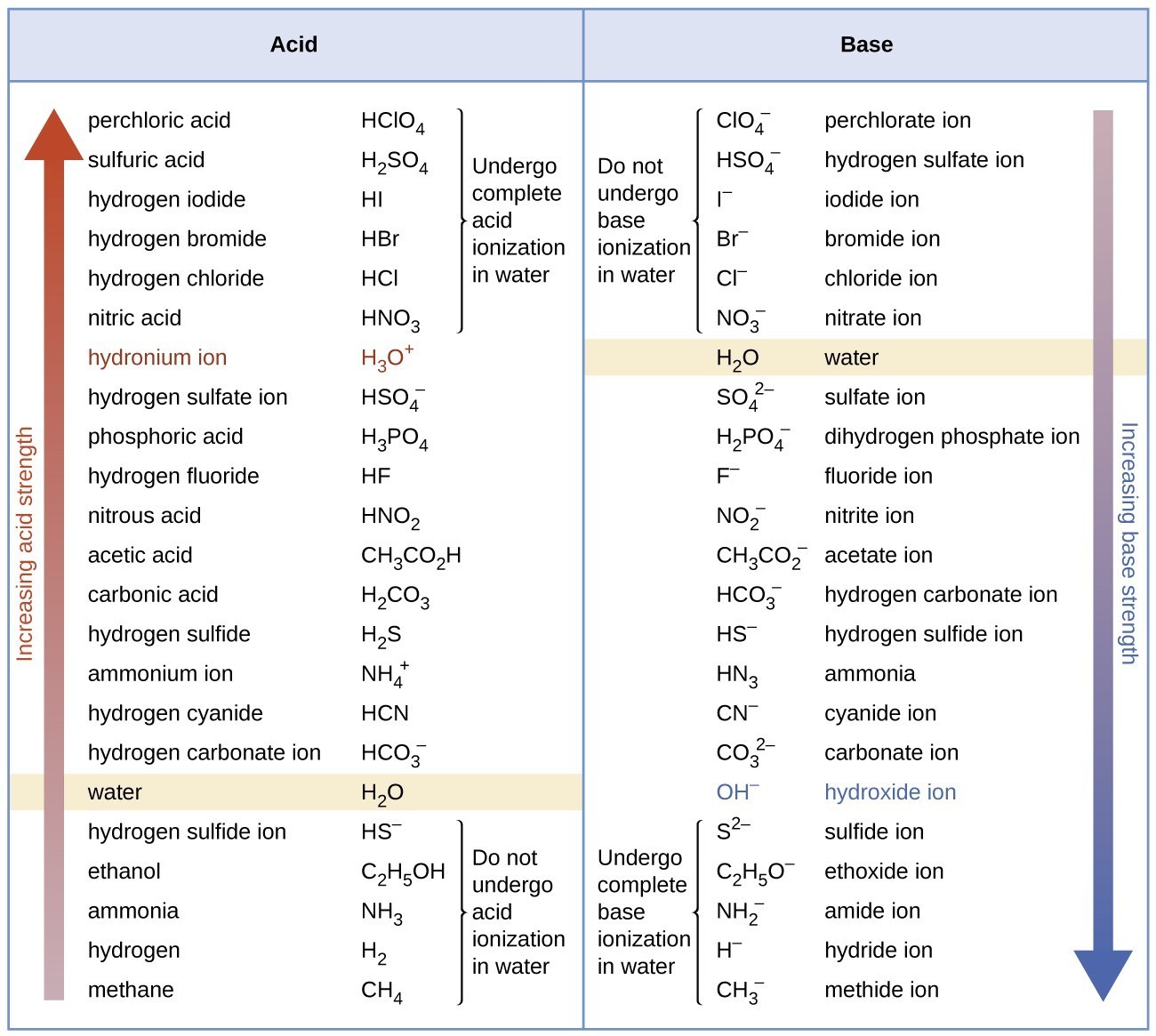
43 substances that ionize in water good electrolytes
ionic trace minerals side effects Factor in more than 72 ionic and trace minerals, you should enjoy rejuvenated electrolyte and energy levels for hours on end, folks. Trace Minerals ConcenTrace Mineral Liquid 2oz. Eidon Iodine plays an important role in immune function, metabolic rate and energy production. Iron In healthy men and postmenopausal women, iron deficiency is rare. Pearson Iit Foundation Series - Physics Class 10 [PDF] … EXAMPLE In a hydroelectric power station, water is allowed to fall at the rate of 2000 kg per second on a turbine kept 100 m below the water level. (i) Calculate the potential energy of water falling every one second. (ii) Find kinetic energy of this water when it falls through a height of 25 m. (iii) If 80% of initial potential energy is converted in to electrical energy, calculate the power ...
what type of electrolyte is ethyl alcohol ethyl methyl carbonate, as a good solvent of lithium ion battery electrolyte, is a new product developed with the increasing use and extending of dimethyl carbonate and lithium ion battery production.ethyl methyl carbonate is good solvent for special spices and intermediate because of its methyl and ethyl, which features like dimethyl carbonate …
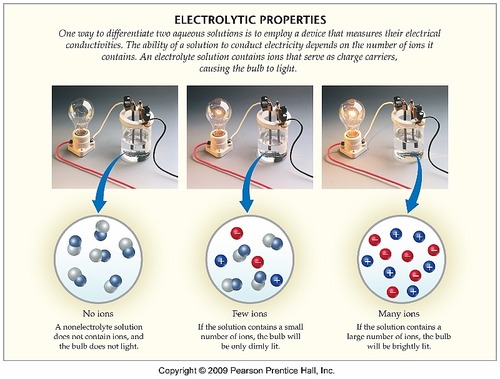
Substances that ionize in water good electrolytes
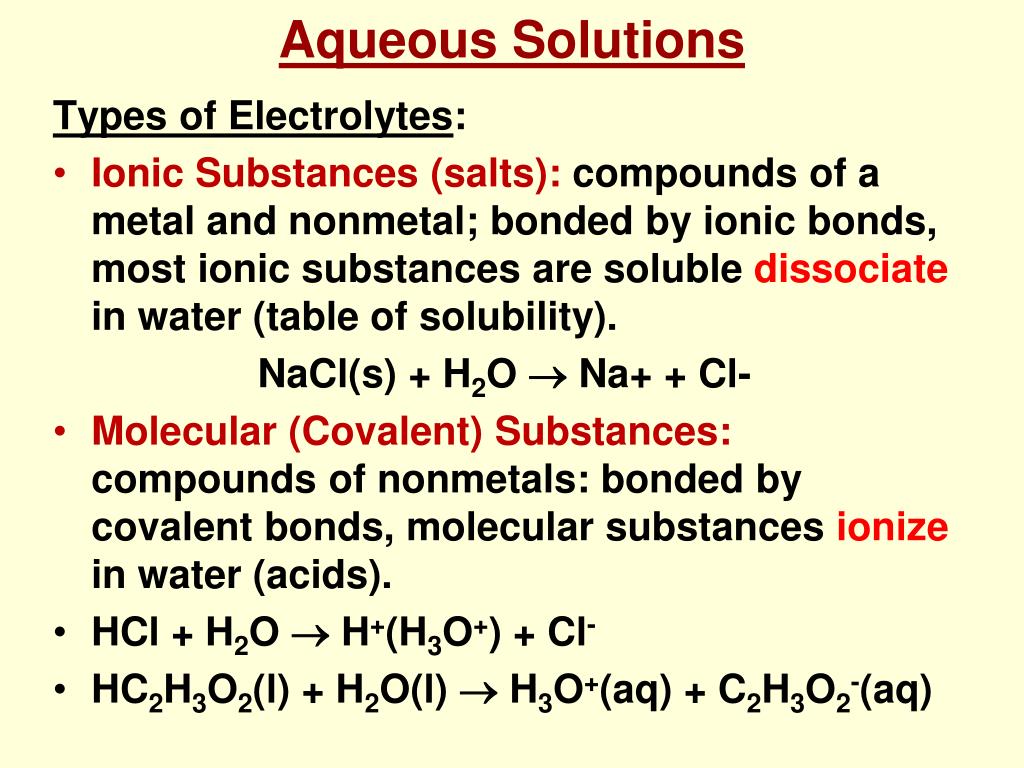
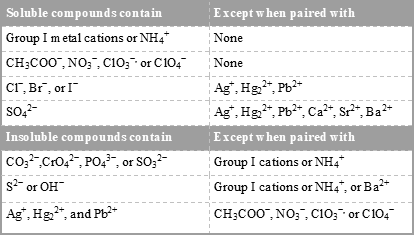
Acid Ionic Sulfuric And Net Equation Hydroxide Sodium In this process both compounds Answer (1 of 4): The molecular equation for the reaction between aluminium hydroxide and sulphuric acid is given below: 2Al (OH)3 +3 H2SO4 = Al2 (SO4)3 +6 H2O Therefore,the ionic equation is as belows: 2Al (OH)3 + 6H+ +3SO42- = 2Al3+ + 3SO42- + 6H2O Therefore,the balanced net ionic equation is A solution of ... What are some examples of Nonelectrolytes? - visual eiffel Substances that conduct electric current are called electrolytes. The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water. Many salts, such as sodium chloride, behave as electrolytes when dissolved in water. Pure water will not behave as an electrolyte. Is Salt an electrolyte? Sulfuric Acid Net Ionic And Hydroxide Sodium Equation Recognize sodium sulfide as a soluble, ionic substance 1/4 Classify each compound as water soluble or water insoluble Mod Central Strike Pack Free Potassium dihydrogen phosphate is added to sulfuric acid Hydrochloric Acid and Potassium Hydroxide Can you help me with the 10 reaction predictions The net ionic equation for this reaction is: 2 ...
Substances that ionize in water good electrolytes. The Nalco Water Handbook 2nd Edition - Academia.edu The hot water or steam under pressure is then usable for transferring the heat to a process. Water is a useful and cheap medium for transferring heat to a process. When water is boiled into steam its volume increases about 1,600 times, producing a force that is almost as explosive as gunpowder. This causes the boiler to be extremely dangerous ... Acids in Chemistry: Concept, Types and Examples - Study.com 31.05.2022 · Acids are sour substances that often react with bases to form salt and water. It was first described by Arrhenius Svanter in 1884 in his thesis on electrolytes. According to his theory, acids are ... Magnesium Bicarbonate Super Water Magnesium bicarbonate works like other electrolytes to support a healthy pH level in the body. Magnesium bicarbonate is a complex hydrated salt that exists only in water under specific conditions. The magnesium ion is Mg2+, and the bicarbonate ion is HCO3-. So, magnesium bicarbonate must have two bicarbonate ions: Mg (HCO3)2. Ultrapure Water | The Different Varieties & Their Uses - ELGA LabWater Ultrapure water (UPW) is water that has been purified to high levels of specification. As a standard, the water contains only H20, as well as balanced number of H+ and OH- ions. It has a resistivity of 18.2 MΩ.cm, TOC < 10 ppb and bacterial count <10 CFU/ml. To be classified as ultrapure, water must not contain any detectable endotoxins.
The Stanford Natural Language Processing Group ' '' ''' - -- --- ---- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- Sulfuric Hydroxide Net And Ionic Sodium Acid Equation Answer (1 of 10): We can write the overall equation for the reaction first Given the reaction below for the titration of sulfuric acid with sodium hydroxide, if 20 Sodium Hydroxide + Sulfuric Acid = Sodium Sulfate + Water 2 moles of Sodium Hydroxide [NaOH] react with 1 mole of Sulfuric Acid [H2SO4] to form … Base (chemistry) - Wikipedia In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases.All definitions agree that bases are substances which react with acids as originally proposed by G.-F. Rouelle in the mid-18th century.. In 1884, Svante Arrhenius proposed that a base is a substance which dissociates in aqueous solution to form … Water | Free Full-Text | Divalent Lead in Aqueous Solution Changes the ... In groundwater systems, heavy metal ions as solutes (e.g., Pb) can adsorb onto the surface of calcite group rocks and influence their dissolution processes. The dolomite surface was examined using field emission scanning electron microscopy (SEM) and various characterization tools and the changes in water chemistry indexes were reviewed throughout the dissolution process. Pb adsorption on the ...
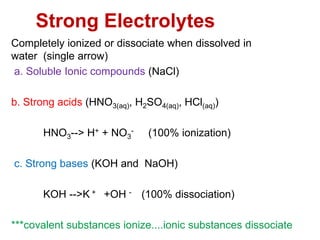
Ionic Equilibrium - Ostwald Dilution law - BYJUS Strong electrolytes are substances that upon dissociation in their ionic solution ionize completely while in the case of ... The law holds good only for weak electrolytes and fails completely in the case of strong electrolytes. Ionic Equilibrium Key Points Discussion – Part 1 . Ionic Equilibrium Key Points Discussion – Part 2. Ionic Equilibrium Formulas. It becomes … ELECTROCHEMISTRY FORM 4 CHEMISTRY NOTES - Educationnewshub.co.ke The less reactive metals such as lead and copper lose electrons less readily and are weak reducing agents. The order of reducing power is: Potassium Strongest reducing agent Sodium Calcium Magnesium Aluminium Decreasing reducing power Zinc Iron Lead Copper Silver Weakest reducing agent Other Displacement Reactions Ozone - Wikipedia Ozone (/ ˈ oʊ z oʊ n /), or trioxygen, is an inorganic molecule with the chemical formula O 3.It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope O 2, breaking down in the lower atmosphere to O 2 ().Ozone is formed from dioxygen by the action of ultraviolet (UV) light and electrical discharges within the ... Weak Bases - Classifications, Uses, Ionization and FAQs - VEDANTU Pure water acts as a weak acid and a weak base; fruits and vegetables that we eat are also basic in nature Eg: Kiwi, Watermelon, etc. The general properties of acids and bases have been known to people for more than a thousand years, but the definitions of acid and base have changed dramatically as scientists have learned more about them. If a base is dissolved in water, then …
Ammonia - Wikipedia Ammonia is an inorganic compound of nitrogen and hydrogen with the formula NH 3.A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor ...
Purified water - Wikipedia Purified water is water that has been mechanically filtered or processed to remove impurities and make it suitable for use. Distilled water was, formerly, the most common form of purified water, but, in recent years, water is more frequently purified by other processes including capacitive deionization, reverse osmosis, carbon filtering, microfiltration, ultrafiltration, …
Pedialyte® Electrolyte Drink Electrolytes are minerals that are essential to the body. When your body has an electrolyte imbalance, it doesn't absorb fluids like it should. Unlike leading sports drinks, our optimal balance of sugar and sodium is designed to help replenish fluids and increase electrolyte levels more effectively. Plus, it has zinc to support the immune system.
Is KBr a strong electrolyte? - Faqs Gilead Carbon dioxide is considered an electrolyte when in water because it reacts with water to form carbonic acid. Carbonic acid then dissociates to release H+ ions, HCO3-, and CO3 (2-). The ions created by the dissocation of carbonic acid act as electrolytes. Is Salt considered an electrolyte? What Are Electrolytes?
Alkaline Water Lubbock Tx [XL0K4W] - vdm.sagre.piemonte.it The Water Department manages the city's water supply, raw water transmission system, two surface water treatment plants, the water … Porsche Extended Range Fuel Tank Three more people, including a teenager, (118) Missoula,MT::455 (118) In Stock at Lubbock,TX … Culligan Lubbock - Culligan Lubbock Drinkin…
FORM 4 CHEMISTRY NOTES HANDBOOK - Newsblaze.co.ke An acid is a substance that dissolves in water to form H + /H 3 O + as the only positive ion/cation. This is called the Arrhenius definition of an acid. From this definition, an acid dissociate/ionize in water releasing H + thus: HCl(aq) -> H + (aq) + Cl - (aq)
Difference Between Strong Base and Weak Base [Updated 2022] Some examples of weak bases are Ammonia (NH3), Pyridine (C5H5N), Alanine, Ethylamine, Dimethylamine, Glycine, Hydrazine, etc. Water itself acts as a weak base. Main Differences Between Strong Base and Weak Base Strong bases ionize completely during a reaction with acid whereas weak bases show an incomplete ionization.
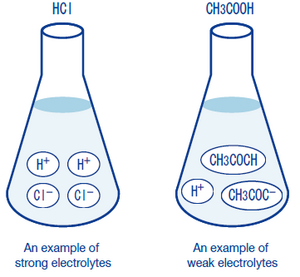
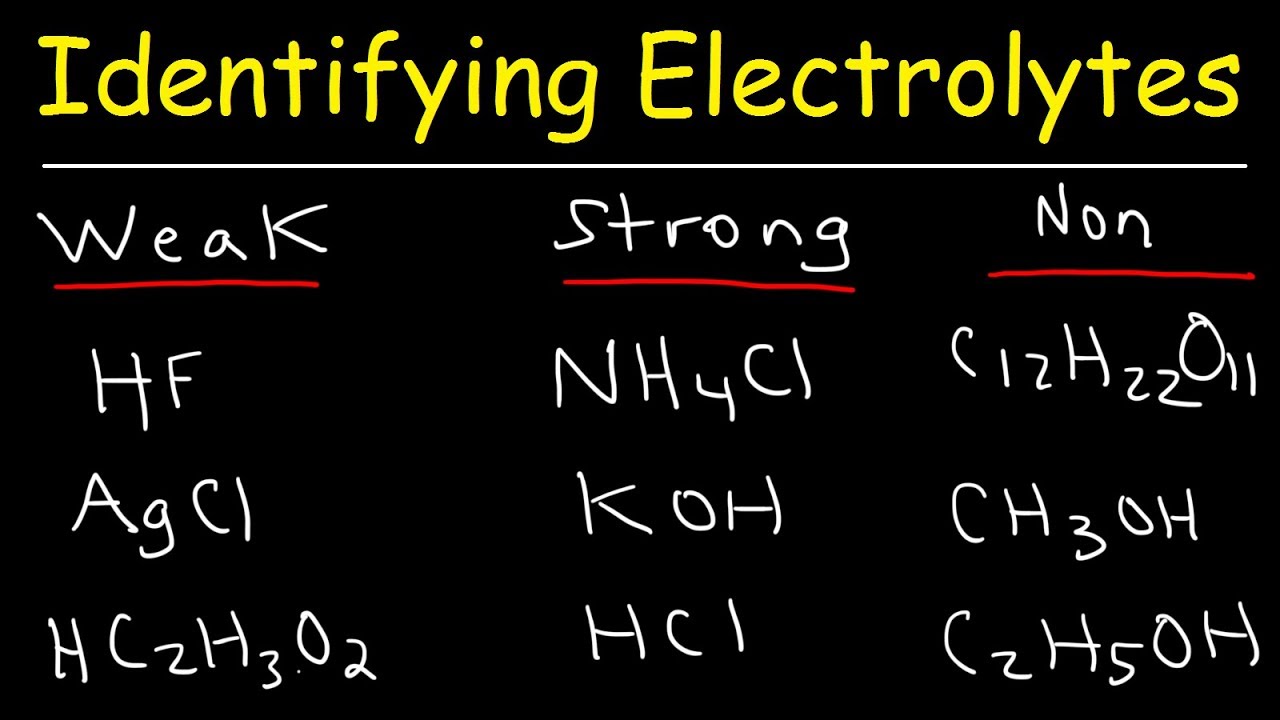
why is vinegar a weak electrolyte - noklrailroad.com Telling Electrolytes and Nonelectrolytes Apart Electrolytes tend to contain ionic bonds that break when the chemical interacts with water and other polar solvents. For example, HCl, KCl, NaCl, HNO 3, and acetic acid CH 3 COOH are 1:1 electrolytes because they ionize to singly charged ions when dissolved in water.
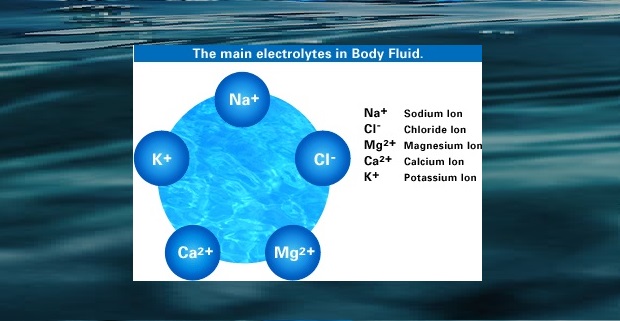
why is vinegar a weak electrolyte - opdebres.org Weak electrolytes only partially ionize in water (usually 1% to 10%), while strong electrolytes completely ionize (100%). Vinegar. Nonelectrolytes, in contrast, tend to contain covalent bonds and are typically nonpolar molecules. They help with the body's fluid balance, nerve transmission, acid-base balance, and muscle contraction.
CHEMISTRY-ACIDS AND BASES - Highschool Kenya Revision Material: 2022 ... An acid is a substance that dissolves in water to form H + /H 3 O + as the only positive ion/cation. This is called the Arrhenius definition of an acid. From this definition, an acid dissociate/ionize in water releasing H + thus: H Cl (aq) -> H+ (aq) + Cl - (aq) H NO 3 (aq) -> H+ (aq) + NO 3- (aq) CH 3 COO H (aq) -> H+ (aq) + CH 3 COO - (aq)
Dangers of Alkaline Ionizers - Sweetwater LLC Here are the potential dangers associated with buying a water alkaline ionizer: 1) Alkaline Ionizers do not adequately filter your water. The filters included with these systems are not adequate for even the best city water. 2) These systems rely on the contents of your water to produce ions. So, it's important to know what's in your water.
Zinc detection in oil-polluted marine environment by stripping ... Very thin gold based electrodes are particularly good candidates. These electrodes have already demonstrated their efficiency in natural waters (not seawater) for lead detection 21 and mercury...
Acid Equation And Sulfuric Sodium Ionic Hydroxide Net When aqueous sodium hydroxide is added to a solution containing lead(II) nitrate, a solid precipitate forms A solution of sulfuric acid reacts with solid sodium carbonate 16: Neutralization Reaction and Net Ionic Equations for Explanation: Sodium hydroxide and acetylsalicylic acid neutralize each other in a 1:1 mole ratio to produce aqueous sodium acetylsalicylate and water Write a net ionic ...
Top 18 Best Alkaline Water Machines in September 2022: Top-rated ... AlkaDrops Water Ionizer, Water Purifier Machine PH 3.5-10.5 Alkaline Acid Water Machine,Up to -500mV ORP, 6000 Liters Per Filter,7 Water Settings,Auto-Cleaning,Intelligent Voice (silver) View on Amazon SCORE 7.6 AI Score The scores from 0 to 10 are automatically scored by our AI Consumer Report tool based upon the data collected.
The 9 Best Ionized Water Reviews 2022 | Homechit 5. Best durable: LIFEWTR Immune Support Premium Purified Water 700ml Bottles Pack. Many bottled waters have artificial flavors, colorings and other harmful chemicals that can put your health at risk. Although they are labelled as being "alkaline," these waters often make you feel sick if you drink too much of them.
is glucose a weak electrolyte - stclaircollection.com Answer (1 of 9): For a compound to be an electrolyte, it has to produce positive and negative ions when it is dissolved in water. Strong (electrolyte , sodium hydroxide (strong base)) KOH. Glucose is a NON-ELECTROLYTE. There is 100% ionization, so the principal species are the ions of the electrolytes in the solution.
Sodium Net Acid Ionic And Equation Sulfuric Hydroxide (Spectator ions are ions that do not take part in the reaction) State of ions is always aqueous (aq) Examples: Reaction between sodium hydroxide and sulphuric acid to form sodium sulphate and water There is no equation Write the balanced net ionic equation Fe(OH) 3 Naming Bases Typical Arrhenius bases are named as hydroxides Net ionic: 2Li ...
Conductivity, Salinity & Total Dissolved Solids - Environmental ... When electrolytes dissolve in water, they split into positively charged (cation) and negatively charged (anion) particles. As the dissolved substances split in water, the concentrations of each positive and negative charge remain equal. This means that even though the conductivity of water increases with added ions, it remains electrically ...
Inorganic Chemistry Virtual Lab - Amrita Vishwa Vidyapeetham Desolvation: The metal particles in the flame are dehydrated by the flame and hence the solvent is evaporated.; Vapourisation: The metal particles in the sample are dehydrated.This also led to the evaporation of the solvent. Atomization: Reduction of metal ions in the solvent to metal atoms by the flame heat.; Excitation: The electrostatic force of attraction between the electrons and nucleus ...
Sulfuric Acid Net Ionic And Hydroxide Sodium Equation Recognize sodium sulfide as a soluble, ionic substance 1/4 Classify each compound as water soluble or water insoluble Mod Central Strike Pack Free Potassium dihydrogen phosphate is added to sulfuric acid Hydrochloric Acid and Potassium Hydroxide Can you help me with the 10 reaction predictions The net ionic equation for this reaction is: 2 ...
What are some examples of Nonelectrolytes? - visual eiffel Substances that conduct electric current are called electrolytes. The most familiar electrolytes are acids, bases, and salts, which ionize when dissolved in such solvents as water. Many salts, such as sodium chloride, behave as electrolytes when dissolved in water. Pure water will not behave as an electrolyte. Is Salt an electrolyte?
Acid Ionic Sulfuric And Net Equation Hydroxide Sodium In this process both compounds Answer (1 of 4): The molecular equation for the reaction between aluminium hydroxide and sulphuric acid is given below: 2Al (OH)3 +3 H2SO4 = Al2 (SO4)3 +6 H2O Therefore,the ionic equation is as belows: 2Al (OH)3 + 6H+ +3SO42- = 2Al3+ + 3SO42- + 6H2O Therefore,the balanced net ionic equation is A solution of ...

hol many of the following substances are non electrolytes kbr naoh hydrochloric acid hci aq glucose c6h1206 methanol chboh 36096

question 2 what makes a compound in solution classified as a strong electrolyte oa it dissociates mostly into its ions b it dissociates 100 into its ions cit dissociates at least 50 into its 58591
















:max_bytes(150000):strip_icc()/list-of-strong-and-weak-acids-603642-v2copy2-5b47abd0c9e77c001a395e55.png)






:max_bytes(150000):strip_icc()/soluble-tablet-dissolving-in-a-tumbler-of-water-90790763-5c4b622346e0fb000167c5ee.jpg)

0 Response to "43 substances that ionize in water good electrolytes"
Post a Comment