45 rate and order of h2o2 decomposition lab answers
PDF Rate and order of h2o2 decomposition lab answers Rate and order of h2o2 decomposition lab answers CHEM 174 Laboratory of Physical Chemistry II Decomposition of Hydrogen Peroxide The composition of our world is controlled by detailed chemical mechanisms and their rates. Extremely complex mechanisms determine the characteristics of everything from living cells to the atmosphere. EOF
Chem 1252 lab report - Shivam Patel Rate of Decomposition for ... - StuDocu Differential rate law, equation for the relationship between the rate of the reaction and its concentration. Using a catalyst for the decomposition reaction, can help to speed up the process but usually it does not end up getting fully used. Procedure For this lab the first task was to 50ml of erlenmeyer flask and fit it with rubber stopper.
Rate and order of h2o2 decomposition lab answers
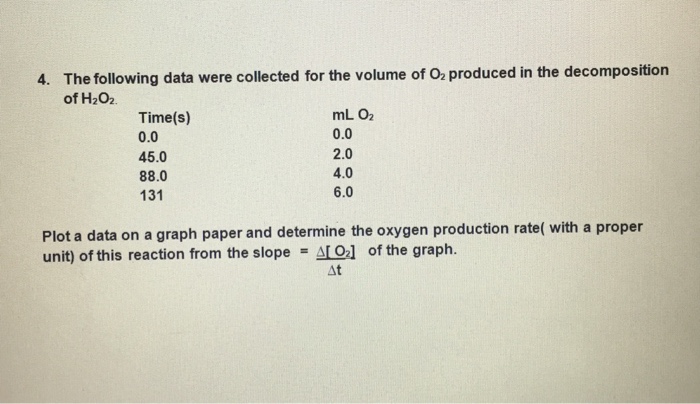
Solved Rates of 30 Pre-lab Chemical Reactions II: Rate and | Chegg.com Rates of 30 Pre-lab Chemical Reactions II: Rate and Order of H2O2 Decomposition Questions Before beginning this experiment in the laboratery, you should be able to answer the fellowing questions What six factors may influence the rate of a reaction? 2 Assume that the rate law for a reaction is eAB ) What is the overall order of the reaction? PDF The Decomposition of Hydrogen Peroxide - Chem21Labs and the initial rate... then the reaction is ___ order with respect to that reactant. triples (31) first increases nine-fold (32) second Decomposition of Hydrogen Peroxide The decomposition of hydrogen peroxide in aqueous solution proceeds very slowly. A bottle of 3% hydrogen peroxide sitting on a grocery store shelf is stable for a long period ... Decomposition Of Hydrogen Peroxide Lab Report - 1278 Words | Cram Kinetic order can be found by using this calculation Rate=k〖 [H_2 O_2]〗^m 〖 [KI]〗^n Also determine the energy of activation. The energy can be calculated by using this equation ln (k_2/k_1 )=- (E_a/R) (1/T_2 -1/T_1 ) Lastly the objective is to understand the molecular components of the rate limiting step of the reaction.
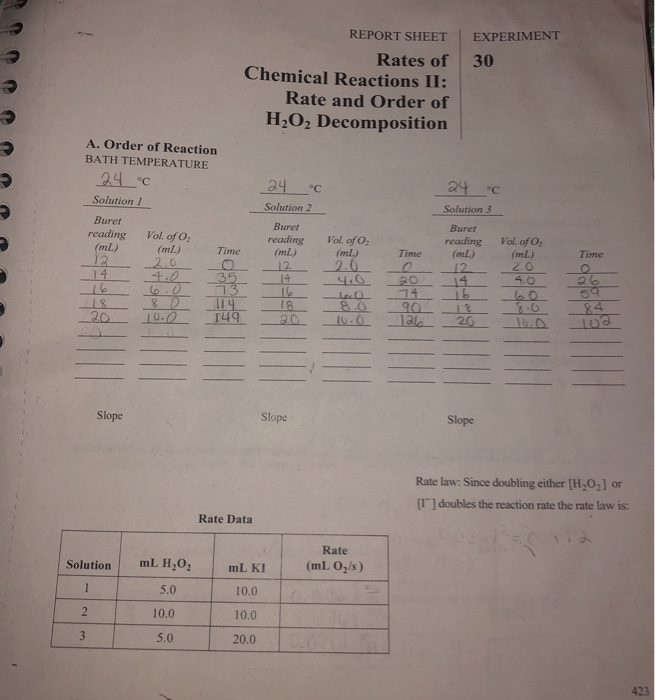
Rate and order of h2o2 decomposition lab answers. Chemistry Lab Report- 3.docx - EXPERIMENT 30. RATE AND ORDER OF H2O2 ... PROCEDURE SOLUTION 1 Add 10.0ml of 0.10MKI and 15.0ml of distilled water to a clean Erlenmeyer flask. Swirl the flask with care for few minutes so that the solution attains the bath temperature in the trough. Add 5.0ml of 3%H2O2and quickly stopper the flask. catalysis - Rate order of decomposition of hydrogen peroxide ... Rate order of decomposition of hydrogen peroxide 2 I have been having some troubles finding a source for my investigation. I am inverstigating the decomposition of hydrogen peroxide, which (according to my teacher/supervisor) is a second order reaction with the use of catalase, and looks like this: R a t e = [ H X 2 O X 2] [ c a t a l a s e] Decomposition of Hydrogen Peroxide Lab Answers - SchoolWorkHelper 1) Calculate the rate constant and write the rate law expression for the catalyzed decomposition of hydrogen peroxide. Explain how you determined the order of the reaction in H2O2 and KI. 12 The following mechanism has been proposed for this reaction: H2O2 + I- -> IO- + H2O (Step 1) H2O2 + IO- -> I- + H2O + O2 (Step 2) Calculations for Rate and Order of H2O2 Lab - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
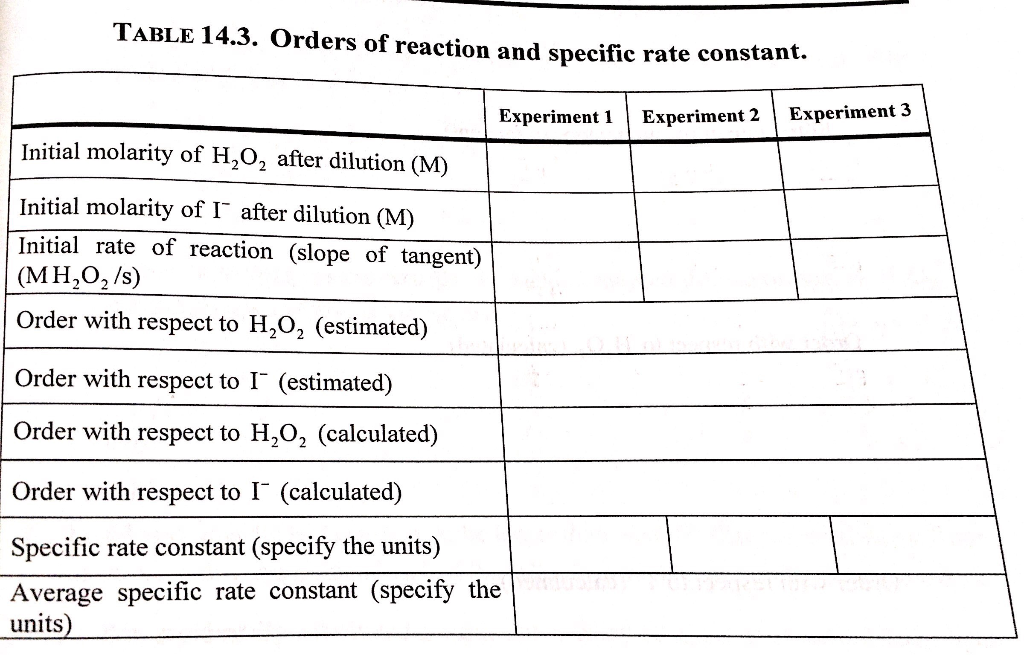
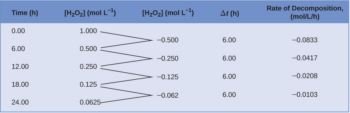
DOC 28. Determination of the Rate of the Decomposition of Hydrogen Peroxide The rate of the rate-determining reaction is calculated as follows: (2) where k1 = the rate constant of the first reaction m = the order of I- in the first reaction n = the order of H2O2 in the first reaction In this experiment, we determine n and m, as well as k1. PDF Decomposition of Hydrogen Peroxide.pdf - SweetStudy A bottle of 3% hydrogen peroxide sitting on a grocery store shelf is stable for a long period of time. The decomposition takes place according to the reaction below. 2 H2O2 (aq) → 2 H2O + O2 (g) A number of catalysts can be used to speed up this reaction, including potassium iodide, manganese (IV) oxide, and the enzyme catalase. Experiment 3 - EXPERIMENT 3 : H2O2 DECOMPOSITION - ORDER AND RATE ... atom experiment h2o2 decomposition order and rate objective: to determine the order and rate of reaction procedure: gas measuring system was assemble as in Solved Rates of chemical reactions II: rate and order of | Chegg.com View the full answer. Transcribed image text: Chemical Reactions II: Rate and Order of H202 Decomposition A. Order of Reaction BATH TEMPERATURE oC oc °C Soluti Buret reading (mL) Buret Buret reading (mL) Vol. of O2 (mL) Vol. of O2 (mL) reading (mL) Vol. of O2 Time Time (mL) Time Slope - Slope - Slope Rate law: Rate Data Rate SolutionmL H202 mL ...
Hydrogen Peroxide Decomposition Lab Report - 1608 Words | Cram Step 5: After the initial rate has been calculated, add 10 micro-liters of catalyst using a micropipette into the hydrogen peroxide. Step 6: Measure the pressure build up of the catalyst with the hydrogen peroxide, again using the gas pressure sensor. Step 7: Constantly stir the hydrogen peroxide with the magnetic stirrer to release oxygen gas ... Decomposition Of Hydrogen Peroxide Lab Report - 1278 Words | Cram Kinetic order can be found by using this calculation Rate=k〖 [H_2 O_2]〗^m 〖 [KI]〗^n Also determine the energy of activation. The energy can be calculated by using this equation ln (k_2/k_1 )=- (E_a/R) (1/T_2 -1/T_1 ) Lastly the objective is to understand the molecular components of the rate limiting step of the reaction. PDF The Decomposition of Hydrogen Peroxide - Chem21Labs and the initial rate... then the reaction is ___ order with respect to that reactant. triples (31) first increases nine-fold (32) second Decomposition of Hydrogen Peroxide The decomposition of hydrogen peroxide in aqueous solution proceeds very slowly. A bottle of 3% hydrogen peroxide sitting on a grocery store shelf is stable for a long period ... Solved Rates of 30 Pre-lab Chemical Reactions II: Rate and | Chegg.com Rates of 30 Pre-lab Chemical Reactions II: Rate and Order of H2O2 Decomposition Questions Before beginning this experiment in the laboratery, you should be able to answer the fellowing questions What six factors may influence the rate of a reaction? 2 Assume that the rate law for a reaction is eAB ) What is the overall order of the reaction?



0 Response to "45 rate and order of h2o2 decomposition lab answers"
Post a Comment