38 substances that dissolve in water
en.wikipedia.org › wiki › LipophilicityLipophilicity - Wikipedia Thus lipophilic substances tend to dissolve in other lipophilic substances, but hydrophilic ("water-loving") substances tend to dissolve in water and other hydrophilic substances. Lipophilicity, hydrophobicity, and non-polarity may describe the same tendency towards participation in the London dispersion force , as the terms are often used ... Substance That Releases Hydrogen Ions When Dissolved In Water A substance that tends to release hydrogen ions when dissolved in water is called a Arrhenius acid. What produces hydrogen ions in water? Hydrogen ions are spontaneously generated in pure water by
Substances That Release Ions When Dissolved In Water Are Called What types of substances dissolve in water? Sugar sodium chloride and hydrophilic proteins are all substances that dissolve in water. Oils fats and certain organic solvents do not dissolve in water because they are hydrophobic. Do ionic substances dissolve in water? Water typically dissolves many ionic compounds and polar molecules.
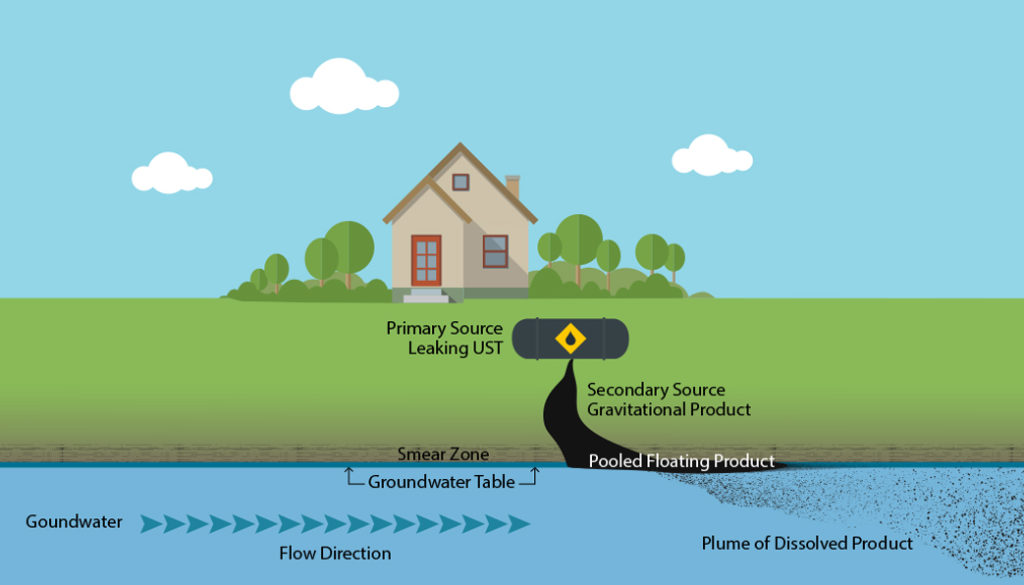
Substances that dissolve in water
⚗️Which type of substance is water able to dissolve? nonpolar ionic ... Water is a liquid that is transparent or colourless, tasteless and odourless which form the Earth hydrosphere and can be use for fringing, cooking, washing, feeding animals and so on. What is Polar Substance? Polar or ionic solvents dissolve polar or ionic solutes. Polar Substance are substances that can dissolve in water completely or polar ... › why-is-water-the-universalWhy Is Water the Universal Solvent? - ThoughtCo Sep 09, 2019 · Water is called the universal solvent because more substances dissolve in water than in any other chemical. This has to do with the polarity of each water molecule. The hydrogen side of each water (H 2 O) molecule carries a slight positive electric charge, while the oxygen side carries a slight negative electric charge. This helps water ... Why do polar substances dissolve in water? Answer: The Are... - Online ... Why do polar substances dissolve in water? Answer: The Are hydrophilic and their polarity is stronger thanwater so it breaks making the water surround the hydronic bonds… Show more Science Biology Share QuestionEmailCopy link This question was created from Chapter 2 Questions Fall 2019.docx Comments (0)
Substances that dissolve in water. › exploring-which-solidsWhich Solids Dissolve In Water - Cool Science for Kids Nov 17, 2011 · When a substance dissolves in water, you can’t see it anymore, it’s still there, but has mixed with the water to make a transparent liquid called a solution. We call substances that dissolve in water soluble. Sugar and salt are examples of soluble substances. Substances that do not dissolve in water are called insoluble. Sand and flour are ... Substances That Will not Dissolve in Water - ScienceBriefss.com Despite being known as the "universal solvent," some substances will never dissolve in water, no matter how hard you try. Density and Dissolution - Water is a solvent, meaning it is a liquid that dissolves substances. Any substance that dissolves is called the solute, and the mixture created when the solvent and solute completely combine and do not separate is called a solution. What of the following substances when dissolve in water ... - Brainly Substances that dissolve in water to form electrically conducting solutions are electrolytes. Substances that dissolve to form nonconducting solutions are known as nonelectrolytes. pls give me brainliest ok Advertisement Advertisement New questions in Science. 1. Which of the following is not correct about waves? What Must Be True For A Substance To Be Able To Dissolve In Water ... The solute is the substance that dissolves. The solvent is the dissolving medium. See also what is a terminating decimal in math What substance dissolves it which is solvent? Solute Solute and solvent A solute is the substance that dissolves to make a solution. In salt solution salt is the solute.
Do Polar and nonpolar molecules dissolve in water? - Webnews21 Water can dissolve in polar or nonpolar substances if we speak about that, so you have to understand and read all articles to get your answer. Hence, the answer is that water is a polar substance, so it can easily mix with polar substances, but it is weak in combining with a nonpolar sense . what substances when dissolved in water will conduct electricity (2022 ... Ionic substances, also known as salts, conduct electricity when dissolved in water or when in the liquid state (molten). Sometimes, when two different solutions are mixed, the substances they contain react with each other and form ions. Hydrophilic Molecules Examples & Interaction - Study.com A hydrophilic molecule is a molecule that can mix and interact with water. Some examples of hydrophilic molecules that can be found in everyday life include: Salt Sugar Amino acids Bleach These... Soluble Substances: Which Solids Dissolve In Water? - Thư Viện Tài ... The Difference Between Soluble Substances and Insoluble Ones . A soluble substance is one that dissolves in a fluid, normally water. It might look like it 's plainly vanish, but in fact, it 's still there - it 's just mixed in to form a liquid called a 'solution '. The solid that dissolves is called the 'solute '.
Solubility table - Wikipedia The table below provides information on the variation of solubility of different substances (mostly inorganic compounds) in water with temperature, at one atmosphere pressure.Units of solubility are given in grams per 100 millilitres of water (g/100 mL), unless shown otherwise. The substances are listed in alphabetical order. Contents Which compound is insoluble in Water? - Learn about chemistry The compounds that are insoluble in water are sulphates of Barium, Strontium and Lead Example Barium sulphate (Bariou tetraoxosulphate Vi) BaSO 4, Strontium sulphate (Strontium tetraoxosulphate Vi) SrSO 4, Lead II teraoxosulphate VI (PbSO 4 ). Calcium sulphate is partially insoluble and partially soluble in water. Types of Drinking Water Contaminants | US EPA The Safe Drinking Water Act defines the term "contaminant" as meaning any physical, chemical, biological, or radiological substance or matter in water. Therefore, the law defines "contaminant" very broadly as being anything other than water molecules. Drinking water may reasonably be expected to contain at least small amounts of some contaminants. Properties of water - Wikipedia Water (H2 O) is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an inherent hint of blue.It is by far the most studied chemical compound and is described as the "universal solvent" and the "solvent of life". It is the most abundant substance on the surface of Earth and the only common substance to exist as a solid ...
en.wikipedia.org › wiki › AlkalinityAlkalinity - Wikipedia Alkalinity (from Arabic: القلوي, romanized: al-qaly, lit. 'ashes of the saltwort') is the capacity of water to resist acidification. It should not be confused with basicity, which is an absolute measurement on the pH scale.
TDS in Water: What it is & How to Calculate - Water Defense Aqueous solubility: carbon dioxide (CO), sulfate, chloride, potassium chloride, heavy metals, and radon can dissolve in water; they are present in small amounts that are usually not harmful to human health because they are rapidly removed from drinking water by filtration or activated carbon treatment.
› special-topics › water-science-schoolConductivity (Electrical Conductance) and Water Completed - USGS Jun 06, 2018 · Water is a most excellent solvent. It doesn't matter if the water comes out of your kitchen faucet, is in a swimming pool or dog dish, comes out of the ground or falls from the sky, the water will contain significant amounts of dissolved substances, minerals, and chemicals. These things are the solutes dissolved in water.
› Dissolve-SaltHow to Dissolve Salt in Water: 9 Steps (with Pictures) - wikiHow May 20, 2022 · The purer the water (i.e. the less contaminants it contains) the more salt you will be able to dissolve into it. This is because there are fewer water molecules interacting with other substances in the water, and therefore, more water molecules free to dissolve the salt.
Solubility of a Compound in Water - Video & Lesson Transcript - Study.com Ions form in water when some compounds dissolve in water. These ions have positive or negative charges, which help them become part of the solution. For example, table salt (NaCl) dissolves into ...
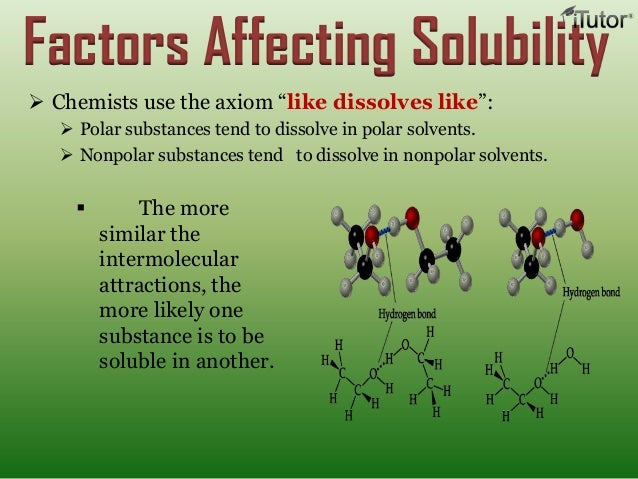
› chemlab › chm1046courseThe Solution Process - Department of Chemistry & Biochemistry Polar substances are likely to dissolve in polar solvents. For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. Nonpolar substances are likely to dissolve in nonpolar solvents. For example, nonpolar molecular substances are likely to dissolve in hexane, a common nonpolar solvent.
Water as a Solvent: Properties, Advantages, Roles & Examples - Embibe Exams Water as a Solvent. Water is a colourless, odourless and tasteless liquid that is essential for the life of all living things on the planet. Because water makes up \(71\%\) of the Earth's surface, the Earth is also known as the "blue planet." \({{\rm{H}}_2}{\rm{O}}\) is the chemical formula for water, and covalent bonds exist between the hydrogen and oxygen atoms in the water molecule.
How Do You Show That A Substance Is Dissolved In Water When Writing An ... The solute is the substance that dissolves. The solvent is the dissolving medium. How do you know if it is aqueous or solid? You can usually tell if something is solid or gas by looking through the problem you are doing (it is normally given) and it is usually marked on the periodic table.
The Chemistry Of Natural Waters - Environmental Chemistry - Dr. Darrin Lew The most common substance oxidized by dissolved oxygen in water is organic matter having a biological origin, such as dead plant matter and animal wastes. If, for the sake of simplicity, the organic matter is assumed to be entirely polymerized carbohydrate (e.g., plant fiber) with an approximate empirical formula of CHzO, the oxidation reaction ...
📐Why do some substances dissolve in water, but other substances do not ... Why do some substances dissolve in water, but other substances do not? 1 See answer Advertisement Advertisement swegster is waiting for your help. Add your answer and earn points. shakilhussain2022 shakilhussain2022 Answer: It has to do with the polairity of said molelule. If you have vegetable oil, for example, it will not disolve in water ...
Why do polar substances dissolve in water? Answer: The Are... - Online ... Why do polar substances dissolve in water? Answer: The Are hydrophilic and their polarity is stronger thanwater so it breaks making the water surround the hydronic bonds… Show more Science Biology Share QuestionEmailCopy link This question was created from Chapter 2 Questions Fall 2019.docx Comments (0)
› why-is-water-the-universalWhy Is Water the Universal Solvent? - ThoughtCo Sep 09, 2019 · Water is called the universal solvent because more substances dissolve in water than in any other chemical. This has to do with the polarity of each water molecule. The hydrogen side of each water (H 2 O) molecule carries a slight positive electric charge, while the oxygen side carries a slight negative electric charge. This helps water ...
⚗️Which type of substance is water able to dissolve? nonpolar ionic ... Water is a liquid that is transparent or colourless, tasteless and odourless which form the Earth hydrosphere and can be use for fringing, cooking, washing, feeding animals and so on. What is Polar Substance? Polar or ionic solvents dissolve polar or ionic solutes. Polar Substance are substances that can dissolve in water completely or polar ...







/white-sachet-with-crystalline-powder-stirred-into-water-glas-480982211-5acb6bb5119fa80037f3a6db.jpg)

0 Response to "38 substances that dissolve in water"
Post a Comment